DocuSign Standard is the UF approved method for securely signing and initialing general business-related electronic documents.
DocuSign for Life Sciences 21 CFR Part 11 is a separate instance specifically and strictly for those involved in health-related Human Subject research who need to route FDA regulated documents and signatures. If you want to send documents for electronic signatures as well as create, edit, and share UFDocuSign document templates in the 21 CFR Part 11 instance of DocuSign, you will need the UF_N_DOCUSIGN_CFR21_11_AUTHOR role.
21 CFR Part 11 contains the FDA’s regulations for electronic documentation and electronic signatures. It outlines the administration of electronic records in a health-related human subjects research’s quality management system. While there is an unlimited license at UF to use DocuSign Standard, DocuSign for Life Sciences 21 CFR Part 11 has only 10,000 envelopes available over a 3-year period. As such, DocuSign 21 CFR Part 11 should ONLY be used for signing and initialing FDA regulated documents.
How To Switch Accounts
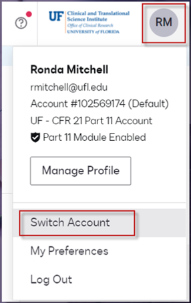
After securely logging into DocuSign, you can switch instances by clicking the identifying icon in the upper right corner of the screen and choosing Switch Account. Only those who are affiliated with human subject research correspondence will have CFR 21-11 as another available account. If you only have 1 account, the switch account option will be inactive. Docusign Add-in for Microsoft 365
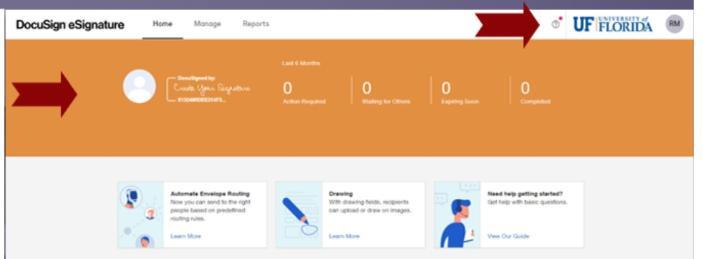
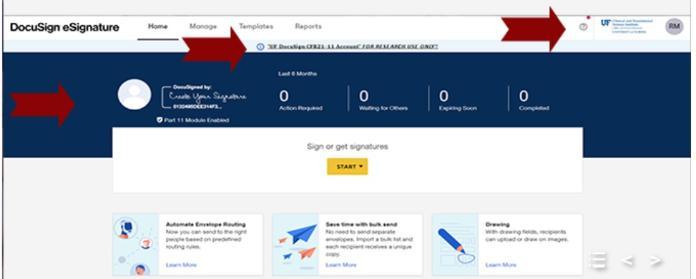
You can easily tell which instance you are in by the look of the screens.
DocuSign Standard will display an orange banner and the UF logo.
DocuSign 21 CFR Part 11 will display a blue banner, the CTSI logo, and a warning message stating “UF DocuSign CFR211-11 Account FOR RESEARCH ONLY!!”
How To Set a Default Account
Follow the instructions on this site: https://support.docusign.com/en/guides/ndse-user-guide-switch-accounts
